

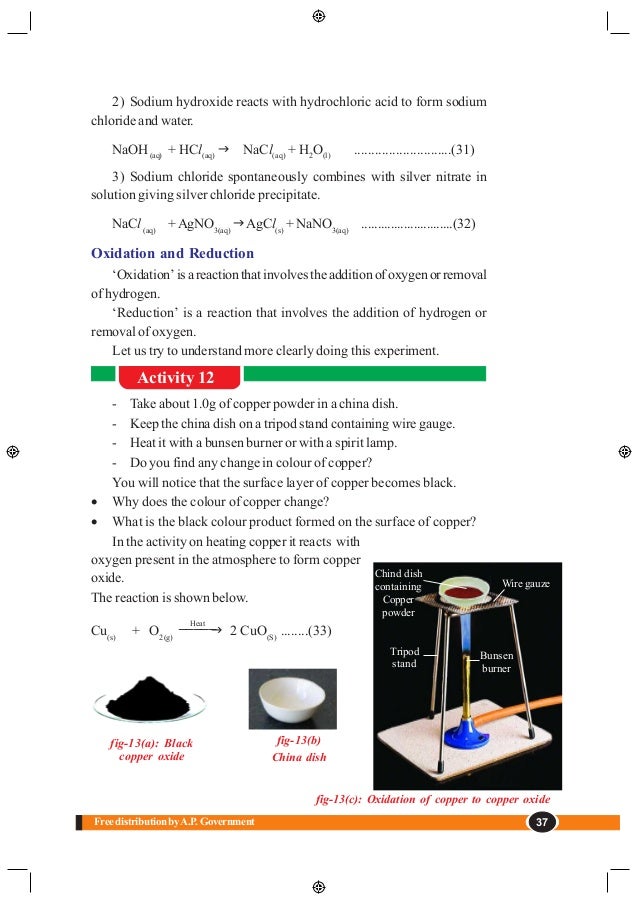
The energy transferred or released when 1 gm of water at 0° C freezes to ice at 0° C. Latent heat of fusion of ice (L) = 80 cal/gm ∴ Total energy released = 540 + 100 = 640 cal.Ĭ) How much energy is released or absorbed when 1 gm of water at 0° C freezes to ice at 0° C? m = 1 gm.ĭifference in temperature = 100-0 = 100☌. The amount of heat energy released when 1 gm of boiling water at 100☌ condenses to water at 100 ☌. Latent heat of vapourisation = 540 cal/gm The amount of heat energy released when 1 gm of boiling water at 100☌ condenses to water at 100☌ī) How much energy is transferred when 1 gm of boiling water at 100° C cools to water 0° C? Latent heat of vapourisation = 540 cal/gm. So surrounding air becomes warm when vapour phase of H20 condenses.Ī) How much energy is transferred when 1 gm of boiling water at 100☌ condenses to water at 100☌?.This energy has to go (somewhere) to the surroundings.When vapour condenses, it changes from gas to liquid.Gases have more higher energy than liquids and solids.The boiling depends on atmospheric pressure.ĭoes the surrounding air become warm or cool when vapour phase of H 2O condenses? Explain. The evaporation depends on surface area, wind, speed, humidity.ĥ. The kinetic energy of the molecules increases with the increase of temperature.ĥ. The temperature of liquids increases up to a constant temperature.Ĥ. Boiling takes place at a definite temperature.ģ. Evaporation takes place at any temperature.Ģ. This temperature is called boiling point of liquid.Ģ. The process in which the liquid phase changes to gaseous phase at constant temperature. The process of escaping of molecules from the surface of a liquid at any temperature is called evaporation.ġ. What are those two processes? Distinguish between those two processes.ġ. Whereas Ramu observed formation of bubbles on a water surface when it is heated. Sita observed decrease in quantity of spirit kept in a vessel placed in open air. Write the differences between evaporation and boiling. So dew is formed on the surface of cold soft drink bottle.Then the molecules of air lose their kinetic energy which leads to lower the temperature and they convert into droplets.During the motion of water molecules in air strike the surface of cold drink bottle.Air contains molecules in the form of vapour.When cold soft drink bottle is kept in open air, the temperature of surrounding air is higher than the temperature of cold drink bottle.What is the reason for the formation of droplets? Raju observed small droplets of water outside a cold soft drink bottle kept in open air. Why do we get dew on the surface of a cold soft drink bottle kept in open air? (AS1) So by panting the water on the tongue undergoes evaporation resulting in the cooling of the dog’s body.

They have sweat glands only in their feet. Since evaporation is a cooling process human body becomes cool.
Physical science projects for 10th class skin#
The temperature of the skin becomes higher and the water in the sweat glands starts evaporating.During summer the temperature in the human body increases.Temperature of a system falls during evaporation.
This cooling effect is due to evaporation. Dogs pant during hot summer days and get their body cooled.How do dogs cool their body? Explain by using the process of evaporation.
What would be the final temperature of a mixture of 50 g of water at 20° C temperature and 50 g of water at 40° C temperature? (AS1)Įxplain, why dogs pant during hot summer days using the concept of evaporation. AP State Syllabus SSC 10th Class Physics Solutions 1st Lesson Heat 10th Class Physics 1st Lesson Heat Textbook Questions and Answers AP State Board Syllabus AP SSC 10th Class Physics Solutions Chapter 1 Heat Textbook Questions and Answers.


 0 kommentar(er)
0 kommentar(er)
